|
Amino linkers can be employed to conjugate biotin, fluorescein or other
modifiers and reporter groups to the 5'end of oligonucleotides, or to
attach oligonucleotides to surfaces.
Proligo offers 2 monomethoxytrityl-protected amino linkers as well as 2 trifluoroacetyl-protected amino linkers.
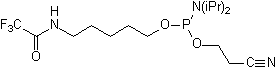
- Trifluoroacetyl (TFA)-protected pentyl (C5) amino linkers amino linker
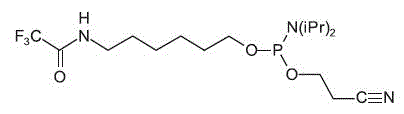
- Trifluoroacetyl
(TFA)-protected pentyl (C6) amino linkers amino linker
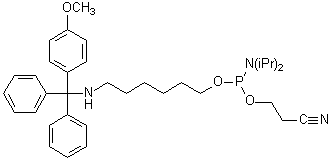
- Monomethoxytrityl
(MMT)-protected amino linker
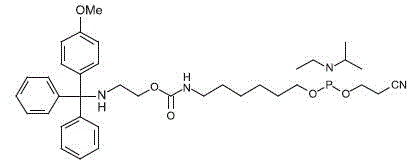
- ssH-linker:
rhe next gereration of MMT linker
Monomethoxytrityl (MMT)-protected amino linker
The MMT-group can be cleaved on the synthesis instrument with acidic
deblock solution to enable on-support labeling protocols.
Alternatively, the MMT-amino linker can be attached in the tritylon mode
of the instrument to provide a purification handle similar to the DMT-group
of conventional ligonucleotides. The MMT-group is then removed with
aqueous acid after purification with either RP-HPLC or a purification
cartridge.
Trifluoroacetyl (TFA)-protected amino linker
The base-labile TFA-group is easily removed with concentrated ammonia
during the cleavage and deprotection step. Additional deprotection steps
are not necessary.
ssH linker
ssH-linker
comprises an internal carbamate group,which is attached to the MMT-protected
amino
group
via a short spacer. The carbamate molety facilitates
the cleavage of the MMT-group under
mildly
acidic conditions while increasing the stability of the MMT-group during
the deprotection
of
the oligonucleotide with ammonia. The
the
amino group through a neighbor group effect, and thereby accelerates
conjugations to aminoreactive
modifiers
and reporters.
The
MMT-group of ssH-linker serves as an excellent purification handle after
the oligonucleotide synthesis in trityl-on mode, similar
to
the DMT-group of conventional oglionucleotides. The MMT-group is cleaved
under very mild conditions in aqueous acetic acid (10% glacial acetic acid
in water, 20 min. room temp.) Alternatively, the MMT-group can be cleaved
on the
synthesis
instrument with acidic deblock solution to enable on-support labeling
protocols.
Key Features of Amino Linkers
- Completely soluble in acetonitrile
- Proligo offers base-labile or acid-labile protecting groups on the
amino linker, depending upon the application.
- Amino linker products are coupled with standard synthesis protocols,
identical to the coupling of DNAmonomer phosphoramidites.
- No change in auxiliary synthesis reagents is required.
- MMT-amino linker features a lipophilic group, which aids in
purification of the modified oligonucleotide after the synthesis.
- The acid-labile MMT-group permits the colorimetric determination of
the coupling efficiency.
- It is recommended to deprotect MMT-amino linker oligonucleotides in
concentrated ammonia at a lower temperature, e.g. at 40°C for 24
hours.
- MMT-group can be removed in aqueous solution with 80% acetic acid at
room temperature for 3 hours.
- TFA-amino linker is completely deprotected with concentrated
ammonia. Additional deprotection steps are not necessary.
Key
features of ssH-linker:
- Advantages over conventional amino linkers
- Deprotection of the amino-group under exceptionally mild conditions
which avoid
depurination side reaction
- Deprotection with 10% aqueous acetic acid at ambient temperature for 20
minutes
- Better labeling efficiency than C6-amino linkers
- Higher coupling efficiency than C6-amino linkers
- Better trityl-on purification efficiency
- Excellent coupling efficiency
- Used with standard deblock, activator, oxidizer and capping-solutions
 |
 |
| MMT-Amino
Linker |
TFA
Pentyl Amino
Linker |
 |
 |
| TFA Hexyl Amino
Linker |
ssH
Linker |
|